 Palestra: Well-defined organometallic precursors for developing tailored catalytic materials.
Palestra: Well-defined organometallic precursors for developing tailored catalytic materials.
Resumo da Palestra: Homo- and hetero-metallic complexes are used as building blocks of well-defined materials, which are employed in small molecules activation (such as H2O, H2 and O2). In this regard, Water splitting is one of the key processes for many applications related to energy storage and conversion. Water oxidation or oxygen evolution reaction (OER) is still considered the mostchallenging step in water splitting since it is a more complex transformation than proton reduction. Cobalt-based metal organic frameworks (MOFs) have recently attracted great interest due to their notable electrocatalytic OER activity and the abundance of this metal in the earth.1,2 Herein, a new cobalt metal−organic framework (2D-Co-MOF) based on well-defined layered double cores that are strongly connected by intermolecular bonds has been developed (Figure 1a). In situ electrochemical activation of a deposited on a graphite electrode produces a well dispersed and ligated nanostructuration of the composite promoting intimate interactions between both components, more abundant electrochemical active sites, where metal centers retain its coordinative chemistry thereby without affecting the intrinsic electrocatalytic properties of the active cobalt centers for OER. The so-activated 2D-Co-MOF@Nafion has been composite exhibits an outstanding electrocatalytic performance for the OER at neutral pH and a high robustness (Figure 1b). The particular coordination chemistryof the MOF consisting of a regular arrangement of multiple Co(II) redox metal sites connectedby appropriate organic ligands can explain the higher catalytic activity of the present MOF.
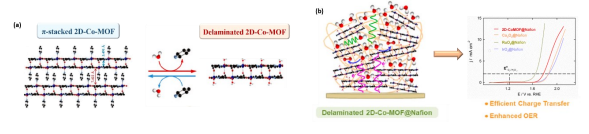 Figure 1. (a) X-Ray structure of 2D Cobalt-MOF and (b) its electrocatalytic OER performance.
Figure 1. (a) X-Ray structure of 2D Cobalt-MOF and (b) its electrocatalytic OER performance.
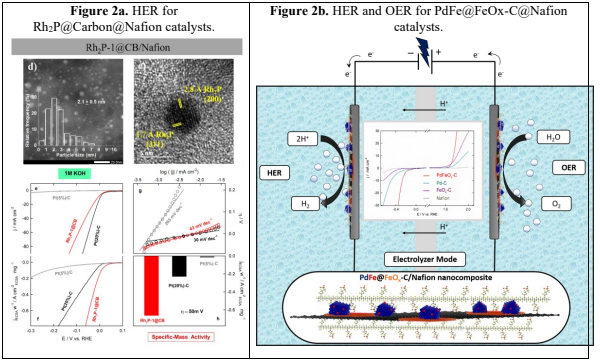
The benchmark HER electrocatalysts are platinum (Pt)-based materials, but these are not goodfor OER, whereas iridium and ruthenium oxides are the state-of-art OER electrocatalysts.Unfortunately, they show insufficient activity toward HER. Indeed, we report a straightforward,efficient and robust synthesis of mono- and bimetallic nanoparticles, promoted through acommercially available metallic source. For instance, the Wilkinson complex in order toprepare Rh2P nanoparticles, which have high crystallinity and are coated with graphitic carbonpatches.3 The resulting materials have been tested in electrocatalytic HER and OER processesat acid, alkaline and neutral media, providing superb HER activity and competitive OERperformance, particularly at neutral and alkaline media, compared to the benchmark HER and OER electrocatalysts Pt and RuOx/IrOx, respectively (Figure 2a).4 Moreover, supported Fe-doped Pd-nanoparticles (NPs) have been prepared via soft transformation of a PdFe-MOF.5 The thus synthesized bimetallic PdFe NPs are supported on, which are essential fordeveloping well-defined and distributed small NPs. The application of this material() as a multifunctional nanocatalyst for the electrocatalytic water splittingprocess has been investigated with promising results, compared with those obtained forpreviously mentioned benchmarking catalysts, in terms of favorable intrinsic activity, wide pHwindow and high stability (Figure 2b).6
 Referências:
Referências:
[1] Gutiérrez Tarriño, S.; Olloqui-Sariego, J. L.; Calvente, J. 1; Palomino, M.; Minguez Espaliargas, G.; Jorda, J. L; Rey, F.; Oña-Burgos, P. Appl. Mat. and Interfaces, 2019, 11, 46658-46665.
[2] Gutiérrez-Tarr-iño, Olloqui-Sariego, J. Calvente, J, J.; Espallargas, Corma, X;
Burgos, P. J. Atn. Chem. soc. 2020, 142, 19198-19208.
[3] Galdeano-Ruano, C.; Corma, A; Oña-Burgos, P. ACS Appl. Nano Mater. 2021, 4, 10743.
[4] Galdeano-Ruano, C.; Olloqui-Sariego, J. L; Oña-Burgos, P. J. Am. Chem. Soc. submitted
[5] Martinez, J.; Mazario, J; Minguez, G. E:, Oña-Burgos, P. Chern. Eur. J. 2020, 26, 13659.
[6] Martinez, J. ; Mazario, J; Olloqui-Sariego, J. L; Oña-Burgos, P. Adv. Sustainable Syst. 2022, 6, 2200096.
Data:
Horário:





